Can wetting balance testing predict plating acceptability?
ENIG was introduced to the industry in the mid-1990s. An electroless means of nickel and gold deposition was desired as a replacement for electrolytic nickel and gold for final finish of high-density circuits. The advantages of ENIG are well-known. In the late 1990s, the issue of black pad was discovered, and chemical suppliers and board manufacturers took steps to reduce or possibly severely limit the occurrence of this phenomenon. In 2002, the publication of IPC-4552, “Performance Specification for Electroless Nickel/Immersion Gold (ENIG) Plating for Printed Boards,” helped steer the industry to establish a more robust ENIG product. Unfortunately, a low level of black pad defects still existed. Determining root causes of field failures was laborious and many times involved heated discussions among board manufacturers, assembly houses, consultants and OEMs regarding root cause of solderability failures from boards with an ENIG
final finish.
More recently, the IPC 4-14 Plating Processes Subcommittee has worked on establishing guidelines for determining acceptable or unacceptable levels of nickel
corrosion, which might be an indicator for a poor soldering nickel surface, from cross-section analysis of bare boards. IPC-4552A was completed in 2017. Unfortunately, some confusion remained regarding determination of Level 1, Level 2 or Level 3 nickel corrosion, and more effort was required by the 4-14 subcommittee for clarification.
The latest ENIG specification revision states that level 2 corrosion must be examined for a continuous intermetallic formed between the nickel layer and bulk solder by dipping the suspect coupon in molten solder for 30 sec. This article discusses the attempt to determine if use of solder wetting balance testing might be able to predict the acceptability of ENIG deposits with level 2 or level 3 corrosion. Solder wetting balance test results after 2x simulated solder reflow conditions were used.
Experimental and Results
The objective was to create level 2 and level 3 product. The latest version of the amendment of IPC-4552A (in progress) describes how to assess the level of corrosion (level 0, 1, 2 and 3). After the assessment is made, seven locations in the worst through-hole or five locations on the worst SMT feature are classified based on corrosion levels. Based on this classification, a “product rating” is extrapolated into Acceptable or Rejectable, with a category in between that may need further evaluation. The product rating is based on a statistical formula for generating a single value for the product from the seven or five evaluations of the corrosion levels found.
To produce a rejectable “product rating,” a highly corroded ENIG sample was needed. In the effort to produce a rejectable product rating, three main variables were explored:
- Type of immersion gold. Three different types of immersion gold were used. The first, “A,” was considered a more aggressive type with a higher deposition rate. The second, “B,” was a milder, high-efficiency product with a more controlled immersion reaction. The third, “C,” was a “reduction-assisted” immersion gold that exhibited minimum corrosion.
- Dwell time in the gold bath (increasing the dwell time beyond vendor specifications). This was based on the concept that increased dwell time in the gold bath is the primary contributor to nickel corrosion.
- Solution agitation in both the electroless nickel and immersion gold baths. This was achieved by using a fast and slow stirring bar in the beakers at the time of plating.
The test vehicle of choice was the solder wetting balance coupons shown in FIGURE 1. The coupons are double-sided boards plated with 25µm of copper.
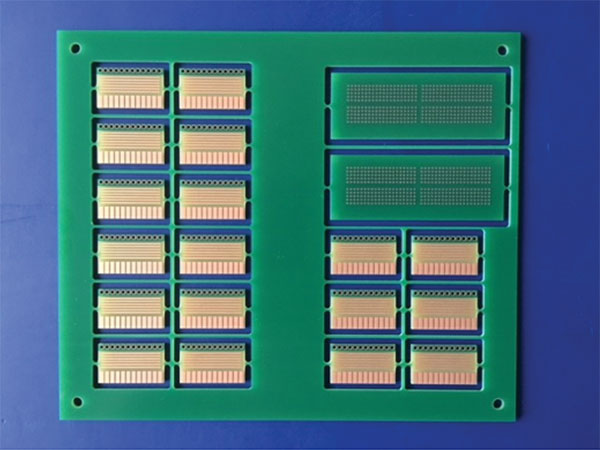
Figure 1. Test vehicle.
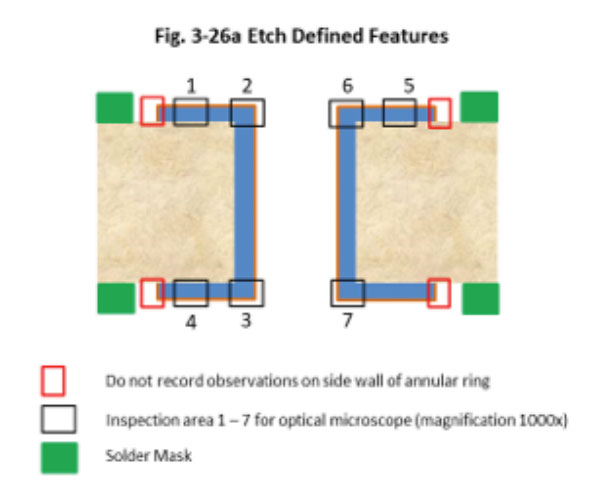
Figure 2. Cross-section (7) location per amended IPC-4552 preview.
Thickness measurements were taken using a Fischer XDV-µ XRF, utilizing a 20-mil collimator with capillary optics. Wetting balance testing was performed according to IPC/J-STD-003 and IPC-4552A specifications, using a Metronelec ST-88 wetting balance tester. Cross-section analysis was performed with epoxy-cured mounts using a standard sequence of grinding and polishing media: 120, 240, 320, 600, 1000 and 2000 SiC grit papers. This was followed by polishing with 0.3µm and 0.05µm Al2O3 media. Cross-section images were taken using a Nikon Epiphot 200 metallograph and JEOL JSM-6010 LA scanning microscope.
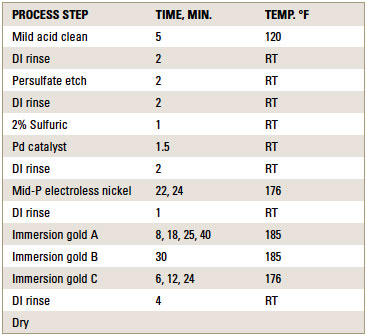
ENIG plating. ENIG plating was performed at 0.2ft.2/gal. bath loading for the electroless nickel solution. Coupons were plated according to the standard ENIG plating sequence shown in TABLE 1.
Table 1. Process Sequence
Results
TABLE 2 depicts the wetting balance results for the variable conditions used in this study. Slight trends were seen for a decrease in wetting time versus solution speed while plating. However, no real differences were seen with final wetting forces. All coupons appeared to wet properly, even the sample with extremely thick gold from immersion gold Solution A. FIGURES 3, 4 and 5 show the wetting balance curves after two reflows, as noted. These are typical of the results seen throughout the testing.
Table 2. Compilation of Deposit Thicknesses and Wetting Balance Results
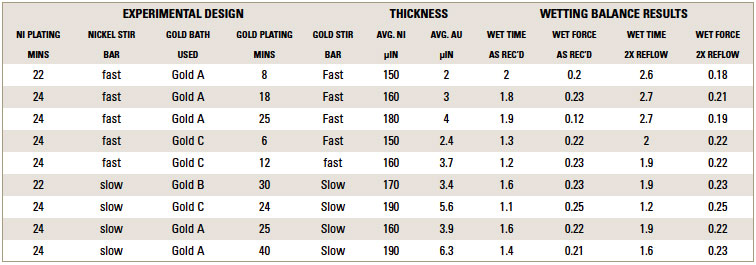
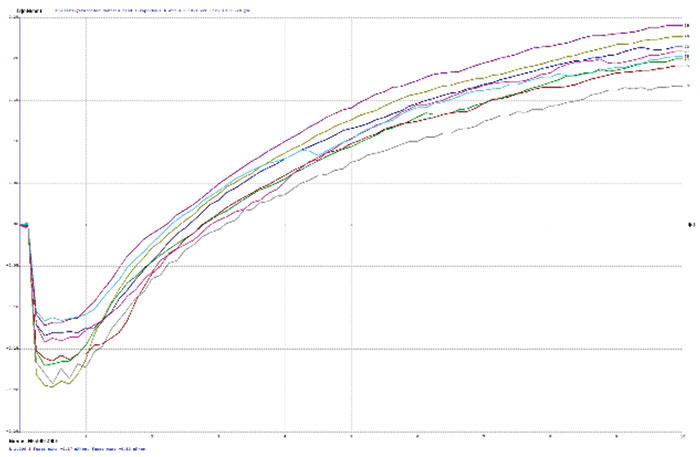
Figure 3. Wetting balance curve after 2x reflow for gold bath A at 4µin with high solution agitation.
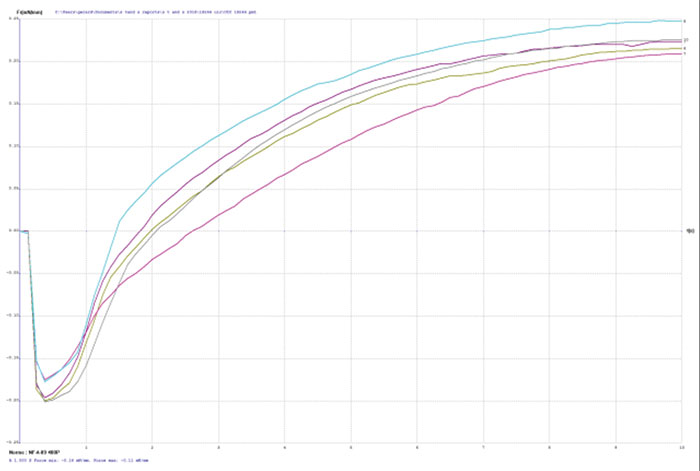
Figure 4. Wetting balance curve after 2x reflow for gold bath B at 3.4µm with low solution agitation.
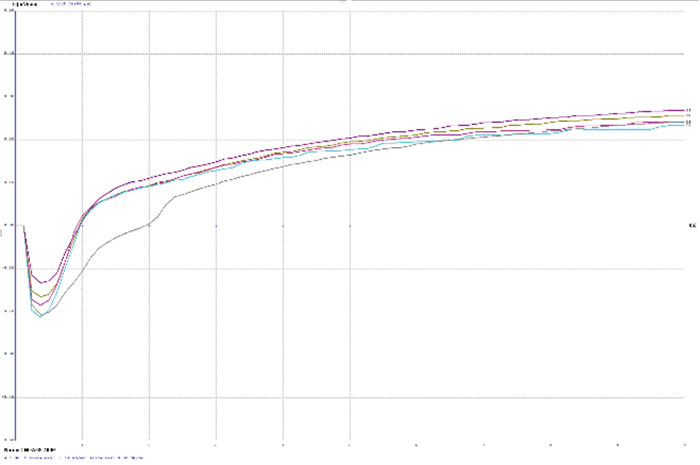
Figure 5. Wetting balance curves after 2x reflow for gold bath C at 5.6µin with low solution agitation.
All parts were examined at 200x for corrosion. The worst sample (maximum dwell time in gold bath A) was examined in detail at 1000x in seven different locations (Figure 2). Each location was assigned a corrosion level. Corrosion evaluation was segmented into three levels as follows, according to a preview of the amended IPC-4552A:
- Corrosion level 1: Number of spikes defects <10, with all spike depth <20% of the nickel thickness. FIGURE 6 shows an example of a corrosion spike.
- Corrosion level 3: Number of spikes >10, and >5 defects penetrate more than 40% of the nickel deposit.
- Corrosion level 2: All other observations.
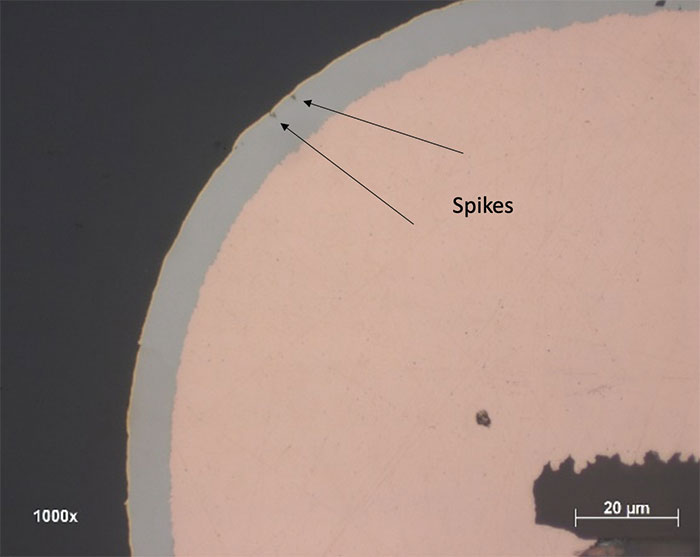
Figure 6. Example of a corrosion spike.
The seven corrosion ratings were tallied to give a product rating according to the following:
- Product rating level 1: >60% of locations show corrosion level 1. This level of hyper-corrosion activity will not degrade solder joint integrity.
- Product rating level 3: >40% of locations show corrosion level 3. This level of hyper-corrosion poses an unacceptably high risk of degraded solder joint integrity.
- Product rating level 2: All other observations are acceptable provided that an acceptable IMC structure is present and visible at 1000x.
Conclusions
- In all the tests run, no “rejectable” product rating was produced. All samples show acceptable. The coupon FIGURE 7 was subjected to a solder dip, and the IMC was examined. The IMC formed was contiguous, indicating an acceptable level 2 corrosion (FIGURE 8).
- Increased dwell time in the immersion gold alone did not produce rejectable product.
- Solution agitation did not influence the degree of
- corrosion.
- The type of gold showed differences, as evidenced by the cross-section analysis. Under the most aggressive dwell time with the more aggressive gold bath A, occasional level 2 and level 3 corrosion was found. The occurrences and frequency of these levels of corrosion were not enough to classify the product as “rejectable.”
- It is worth noting the “reduction-assisted” immersion gold C showed no level 2 or level 3 corrosion on any of the cross-sections evaluated, regardless of dwell time.

Figure 7. Seven cross-sections showing different levels of corrosion and the overall product rating.

Figure 8. Contiguous IMC.
Further Work
Due to time constraints, the authors decided to publish their findings without achieving the objective of determining if wetting balance testing could be a referee for ENIG corrosion acceptability.
Additional work is planned to produce a rejectable product. New variables will be explored, such as the degree of catalyzation and electroless Ni rate of deposition, to produce a compromised nickel deposit susceptible to corrosion during immersion gold deposition.
Ed.: This article was originally published in the SMTA International Proceedings in October 2018 and is republished here with permission of the authors.
Don Gudeczauskas is technical director and George Milad is national accounts manager for technology at Uyemura (uyemura.com); This email address is being protected from spambots. You need JavaScript enabled to view it. and This email address is being protected from spambots. You need JavaScript enabled to view it..